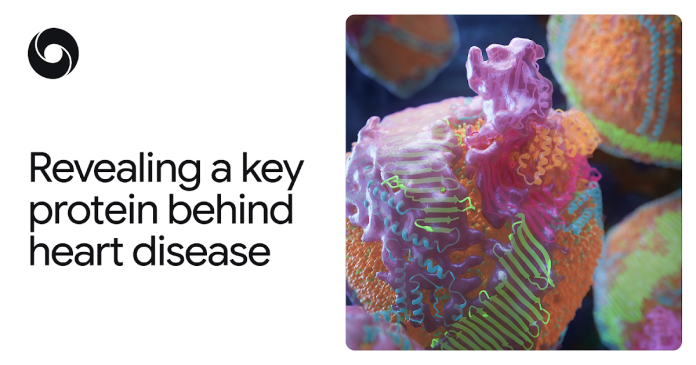
In a breakthrough discovery based on AlphaFold, scientists have mapped the structure of a large protein that gives form to “bad cholesterol” – a discovery that could help change the way researchers and clinicians treat the world's leading cause of death
The race to reveal a key protein responsible for heart disease has long been both an important public health goal and a persistent scientific problem.
For assistant professors Zachary Berndsen and Keith Cassidy of the University of Missouri (Mizzou), it was also personal. They both have a family history of heart disease, which reminds them of what's at stake in their work to better understand this deadly disease and ultimately help treat it.
“For 50 years, people have wanted to see what this protein looks like,” Berndsen says.
This protein, apoB100, failed to map not only because it is huge (for a protein), but also because it binds to fats and other molecules in complex ways. ApoB100 forms the molecular scaffold of “bad cholesterol”, known to scientists as low-density lipoprotein (LDL).
LDL is the main carrier of fat in the bloodstream and a key risk factor for atherosclerotic cardiovascular disease (ASCVD), the leading cause of death worldwide. Discovering the structure of its key protein should shed light on how bad cholesterol becomes harmful in the body, giving scientists a better chance of developing ways to prevent and treat ASCVD. AlphaFold plays a key role in these efforts.
At Mizzou, biochemist Berndsen first used cryo-electron microscopy (cryo-EM) to capture images of LDL particles. The images weren't sharp enough to map the structure of apoB100 with atomic precision, so Berndsen's collaborating physicist Cassidy turned to AlphaFold. He used these to generate atomic-resolution protein structure predictions, and then refined these predicted shapes by comparing them to cryo-EM image data.
Getting to the problem using cryo-EM and Alphafold microscopy made this breakthrough possible, says Cassidy: “AlphaFold played a huge role in this discovery, providing the raw material for interpreting our experimental structure in a way that, quite frankly, was simply not possible before.”
The resulting model revealed in exquisite detail the key protein of bad cholesterol: the cage-like envelope that surrounds each LDL particle, including a ribbon-like band that keeps the particle intact in the bloodstream. Knowledge of this structure opens up new possibilities for preventing, diagnosing, and treating high cholesterol and ASCVD, including therapies that could more precisely target LDL. It is difficult to overestimate the potential benefits to global health.
Although such applications will take time, revealing the structure of apoB100 is a groundbreaking achievement and deeply satisfying for Berndsen. “It was the first structure I checked in AlphaFold the week it became available, and the first protein I wanted to look at with our two-story cryo-EM machine,” he says. “Solving the structure of apoB100 was a dream come true.”