The new AI system designs proteins that are successfully associated with target molecules, with the possibility of drug design, understanding of diseases and more.
Each biological process in the body, from cell growth to immune response, depends on the interaction between molecules called proteins. Like the key to the lock, one protein may be associated with another, helping regulate critical cellular processes. The tools of forecasting protein structure, such as Alphafold, have already given us a huge insight into how proteins interact with each other to perform their functions, but these tools cannot create new proteins for directly manipulating these interactions.
However, scientists can create new proteins that are effectively associated with target molecules. These secrets can help researchers accelerate the progress in a wide spectrum of research, including drug development, cellular and tissue imaging, understanding and diagnosis of diseases – even resistance to pests. One sec Recent approaches to machine learning Protein has made great progress to design, this process is still labor -intensive and requires intensive experimental tests.
Today we present AlphaproteoOur first AI system for designing new binding high -strength proteins to be used as structural elements for biological and health tests. This technology can accelerate our understanding of biological processes and help in discovering new drugs, the development of bosocians and others.
Alphaproteo can generate new protein treads for various target proteins, including VEGF-Awhich is associated with cancer and complications of diabetes. For the first time, the AI tool was able to design a successful protein binder for VEGF.
Alphaproteo also achieves higher experimental indicators of good luck and 3 to 300 times better affordable binding than the best existing methods on seven test target proteins.
Complete learning how proteins are associated with each other
Protein segrenators, which can be closely associated with the target protein, are difficult to design. Traditional methods are intensive temporarily, requiring many rounds of intensive laboratory work. After creating a binder, additional experimental rounds undergo to optimize the affinity of binding, so they are associated enough to be useful.
Trained on large amounts of protein data from Protein data bank (PDB) and over 100 million anticipated structures from Alphafold, Alphaproteo has learned countless ways to bind molecules. Given the structure of the target molecule and a set of preferred locations of binding this molecule, Alphaproteo generates candidate protein, which is associated with the goal in these locations.
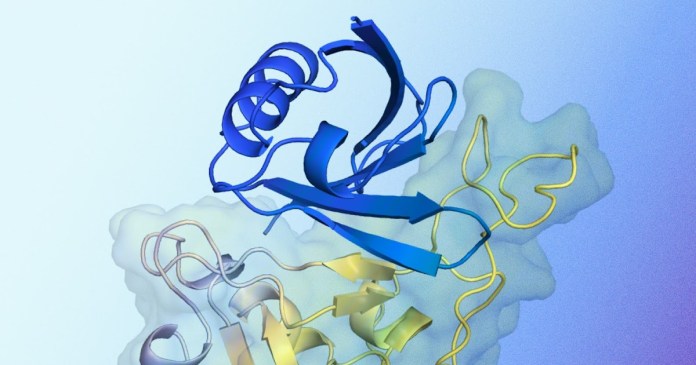
Illustration of the expected structure of the protein binder interacting with the target protein. The blue protein binder structure generated by Alfaproteo, designed for binding with target protein, is shown in blue. Shown in yellow is target protein, in particular the domain binding the SARS-COV-2 receptor
Demonstrating success on important protein binding purposes
To test Alfaproteo, we designed binders for various target proteins, including two viral proteins involved in infection, Bhrf1 AND SARS-DONE-2 Binding domain of the spine protein receptor, SC2RBD and five proteins involved in cancer, inflammation and autoimmune diseases, 7RɑIN PD-L1IN TrackIN IL-17A AND VEGF-A.
Our system has highly competitive indicators of the success of the binding and the best in its class strong binding forces. For seven goals, Alphaproteo generated candidates in-silico proteins, which strongly got involved with their intended proteins after examining experimentally.
The network of illustrations of the anticipated structures of the seven target proteins for which Alfaproteo generated a successful binder. Shown in blue are examples of binders tested in a wet laboratory, shown in yellow protein goals, and highlighted in dark yellow binding areas are intended.
Viral protein for one specific purpose Bhrf188% of our candidate molecules associated with success after testing in Google Deepmind Wet Lab. Based on the tested goals, Alphaproteo binders are also associated on average 10 times stronger than the best existing design methods.
For another purpose, TrackOur binders are even stronger than the best previously designed binders for this purpose that has gone through Many rounds of experimental optimization.
A bar chart showing the experimental indicators of the success of Alphaproteo for each of the seven target proteins, compared to other design methods. Higher indicators of success mean that less projects should be tested to find successful binders.
A bar chart showing the best affinity for Alphaproteo projects without experimental optimization for each of the seven target proteins, compared to other design methods. The lower affinity means that the binder protein is more closely related to the target protein. Pay attention to the logarithmic scale of the vertical axis.
Validation of our results
Apart from in silico We involved validation and testing of Alphaproteo in our wet laboratory Peter Cherpanov'sIN Katie Bentley's AND David Lv Bauer's Research groups with Francis Crick Institute To confirm our protein treads. In various experiments, they dived deeper into some of our stronger SC2RBD and VEGF binders. Research groups have confirmed that the interactions of these binders were actually similar to what Alphaproteo predicted. In addition, groups have confirmed that binders have a useful biological function. For example, it has been shown that some of our SC2RBD binders prevent SARS-COV-2 and some of its variants of cell infection.
Alphaproteo performance indicates that this may drastically shorten the time needed for initial experiments involving binding proteins for a wide range of applications. However, we know that our AI system has restrictions because it was unable to design successful binders compared to the 8th purpose, TNFɑProtein associated with autoimmune diseases such as rheumatoid arthritis. We chose TNFɑ to strongly challenge Alphaproteo, because computing analysis showed that it would be extremely difficult to design binders. We will continue to improve and expand Alphaproteo's capabilities to finally solve such difficult goals.
Achieving a strong binding is usually only the first step in protein design, which can be useful for practical applications, and there is much more bioevement in the process of research and development.
Towards responsible development of protein design
Protein design is a rapidly developing technology that has great potential for the development of science in everything, from understanding factors causing diseases, to accelerating the development of diagnostic tests for virus explosions, supporting more sustainable production processes, and even cleaning pollutants from the environment.
Take into account the potential risk of biological security, based on ours long -term approach to responsibility and security, We work with leading external experts to inform our gradual approach to sharing this work and nutrition of community efforts to develop the best practices, including NTI 'S (nuclear initiative) new AI BIO Forum.
Going further, we will cooperate with the scientific community to use Alphaproteo about influential biology problems and understand its limitations. We also studied its applications in the field of drug design in ISOMORFIC LABS and we are excited to what the future will bring.
At the same time, we are still improving the success rate and the affinity of Alphaproteo algorithms, expanding the scope of design problems that it can solve, and cooperating with scientists in the field of machine learning, structural biology, biochemistry and other disciplines to develop a responsible and more comprehensive offer of protein design for the community.
If you are a biologist whose research could benefit from binding proteins specific to the goal and you want to register interest in being a trusted tester for Alphaproteo, please contact us at alphaproteo@google.com.
We will process messages received in accordance with ours Privacy policy.
Thanks
The research was developed jointly by our Białka Design Team and the WET LAB team.
We would like to thank our colleagues Peter Cherpanov, David Bauer, Katie Bentley and their groups at the Francis Crick Institute for invaluable experimental observations and results, Alphafold team, whose previous work and algorithms provided the training and view of the assessment, as well as many other teams in Google, as well to this program.